About the Journal
The Critical Care Science (Crit Care Sci), ISSN 2965-2774 (formerly Revista Brasileira de Terapia Intensiva), is a continuous publication of the Associação de Medicina Intensiva Brasileira (AMIB) and the Sociedade Portuguesa de Cuidados Intensivos (SPCI) and has the objective to disseminate high-quality clinical, epidemiological, translational, and health services research related to adult and pediatric critical care medicine.
No results found.
-
Original Article
Efficacy of melatonin in decreasing the incidence of delirium in critically ill adults: a randomized controlled trial
Crit Care Sci. 2024;36:e20240144en
Abstract
Original ArticleEfficacy of melatonin in decreasing the incidence of delirium in critically ill adults: a randomized controlled trial
Crit Care Sci. 2024;36:e20240144en
DOI 10.62675/2965-2774.20240144-pt
Views542See moreABSTRACT
Objective:
To determine whether enteral melatonin decreases the incidence of delirium in critically ill adults.
Methods:
In this randomized controlled trial, adults were admitted to the intensive care unit and received either usual standard care alone (Control Group) or in combination with 3mg of enteral melatonin once a day at 9 PM (Melatonin Group). Concealment of allocation was done by serially numbered opaque sealed envelopes. The intensivist assessing delirium and the investigator performing the data analysis were blinded to the group allocation. The primary outcome was the incidence of delirium within 24 hours of the intensive care unit stay. The secondary outcomes were the incidence of delirium on Days 3 and 7, intensive care unit mortality, length of intensive care unit stay, duration of mechanical ventilation and Glasgow outcome score (at discharge).
Results:
We included 108 patients in the final analysis, with 54 patients in each group. At 24 hours of intensive care unit stay, there was no difference in the incidence of delirium between Melatonin and Control Groups (29.6 versus 46.2%; RR = 0.6; 95%CI 0.38 – 1.05; p = 0.11). No secondary outcome showed a statistically significant difference.
Conclusion:
Enteral melatonin 3mg is not more effective at decreasing the incidence of delirium than standard care is in critically ill adults.
-
Original Article
Conscious prone positioning in nonintubated COVID-19 patients with acute respiratory distress syndrome: systematic review and meta-analysis
Crit Care Sci. 2024;36:e20240176en
Abstract
Original ArticleConscious prone positioning in nonintubated COVID-19 patients with acute respiratory distress syndrome: systematic review and meta-analysis
Crit Care Sci. 2024;36:e20240176en
DOI 10.62675/2965-2774.20240176-en
Views241See moreABSTRACT
Objective:
To systematically review the effect of the prone position on endotracheal intubation and mortality in nonintubated COVID-19 patients with acute respiratory distress syndrome.
Methods:
We registered the protocol (CRD42021286711) and searched for four databases and gray literature from inception to December 31, 2022. We included observational studies and clinical trials. There was no limit by date or the language of publication. We excluded case reports, case series, studies not available in full text, and those studies that included children < 18-years-old.
Results:
We included ten observational studies, eight clinical trials, 3,969 patients, 1,120 endotracheal intubation events, and 843 deaths. All of the studies had a low risk of bias (Newcastle-Ottawa Scale and Risk of Bias 2 tools). We found that the conscious prone position decreased the odds of endotracheal intubation by 44% (OR 0.56; 95%CI 0.40 – 0.78) and mortality by 43% (OR 0.57; 95%CI 0.39 – 0.84) in nonintubated COVID-19 patients with acute respiratory distress syndrome. This protective effect on endotracheal intubation and mortality was more robust in those who spent > 8 hours/day in the conscious prone position (OR 0.43; 95%CI 0.26 – 0.72 and OR 0.38; 95%CI 0.24 – 0.60, respectively). The certainty of the evidence according to the GRADE criteria was moderate.
Conclusion:
The conscious prone position decreased the odds of endotracheal intubation and mortality, especially when patients spent over 8 hours/day in the conscious prone position and treatment in the intensive care unit. However, our results should be cautiously interpreted due to limitations in evaluating randomized clinical trials, nonrandomized clinical trials and observational studies. However, despite systematic reviews with meta-analyses of randomized clinical trials, we must keep in mind that these studies remain heterogeneous from a clinical and methodological point of view.
-
Viewpoint
Why the Sequential Organ Failure Assessment score needs updating?
Crit Care Sci. 2024;36:e20240296en
Abstract
ViewpointWhy the Sequential Organ Failure Assessment score needs updating?
Crit Care Sci. 2024;36:e20240296en
DOI 10.62675/2965-2774.20240296-pt
Views238The Sequential Organ Failure Assessment (SOFA) score was developed almost 30 years ago. It rapidly became one of the most widely used scoring systems in intensive care, both for clinical practice and research,(,) and remains one of the most cited scores in our speciality. Since its original description, there have been substantial changes in clinical […]See more -
Letter to the Editor
To: Posterior reversible encephalopathy syndrome in a child with severe multisystem inflammatory syndrome due to COVID-19
Crit Care Sci. 2023;35(4):427-428
Abstract
Letter to the EditorTo: Posterior reversible encephalopathy syndrome in a child with severe multisystem inflammatory syndrome due to COVID-19
Crit Care Sci. 2023;35(4):427-428
DOI 10.5935/2965-2774.20230283-pt
Views143To the editorWe read with interest the article by Dominguez-Rojas et al. about a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR)-negative 9-year-old male who underwent laparotomy for suspected acute abdomen (vomiting, abdominal pain, diarrhea), which was noninformative.() On postoperative day one, the patient experienced respiratory insufficiency attributed to pneumonia with pleural […]See more -
Original Article
Goal-directed therapy guided by the FloTrac sensor in major surgery: a systematic review and meta-analysis
Crit Care Sci. 2024;36:e20240196en
Abstract
Original ArticleGoal-directed therapy guided by the FloTrac sensor in major surgery: a systematic review and meta-analysis
Crit Care Sci. 2024;36:e20240196en
DOI 10.62675/2965-2774.20240196-en
Views77ABSTRACT
Objective
To provide insights into the potential benefits of goal-directed therapy guided by FloTrac in reducing postoperative complications and improving outcomes.
Methods
We performed a systematic review and meta-analysis of randomized controlled trials to evaluate goal-directed therapy guided by FloTrac in major surgery, comparing goal-directed therapy with usual care or invasive monitoring in cardiac and noncardiac surgery subgroups. The quality of the articles and evidence were evaluated with a risk of bias tool and GRADE.
Results
We included 29 randomized controlled trials with 3,468 patients. Goal-directed therapy significantly reduced the duration of hospital stay (mean difference -1.43 days; 95%CI 2.07 to -0.79; I2 81%), intensive care unit stay (mean difference -0.77 days; 95%CI -1.18 to -0.36; I2 93%), and mechanical ventilation (mean difference -2.48 hours, 95%CI -4.10 to -0.86, I2 63%). There was no statistically significant difference in mortality, myocardial infarction, acute kidney injury or hypotension, but goal-directed therapy significantly reduced the risk of heart failure or pulmonary edema (RR 0.46; 95%CI 0.23 – 0.92; I2 0%).
Conclusion
Goal-directed therapy guided by the FloTrac sensor improved clinical outcomes and shortened the length of stay in the hospital and intensive care unit in patients undergoing major surgery. Further research can validate these results using specific protocols and better understand the potential benefits of FloTrac beyond these outcomes.
Keywords:Goalsheart failureIntensive care unitsLength of stayMonitoring, intraoperativeTreatment outcomeSee more -
Clinical Report
Prospective, randomized, controlled trial assessing the effects of a driving pressure–limiting strategy for patients with acute respiratory distress syndrome due to community-acquired pneumonia (STAMINA trial): protocol and statistical analysis plan
Crit Care Sci. 2024;36:e20240210en
Abstract
Clinical ReportProspective, randomized, controlled trial assessing the effects of a driving pressure–limiting strategy for patients with acute respiratory distress syndrome due to community-acquired pneumonia (STAMINA trial): protocol and statistical analysis plan
Crit Care Sci. 2024;36:e20240210en
DOI 10.62675/2965-2774.20240210-en
Views72ABSTRACT
Background:
Driving pressure has been suggested to be the main driver of ventilator-induced lung injury and mortality in observational studies of acute respiratory distress syndrome. Whether a driving pressure-limiting strategy can improve clinical outcomes is unclear.
Objective:
To describe the protocol and statistical analysis plan that will be used to test whether a driving pressure-limiting strategy including positive end-expiratory pressure titration according to the best respiratory compliance and reduction in tidal volume is superior to a standard strategy involving the use of the ARDSNet low-positive end-expiratory pressure table in terms of increasing the number of ventilator-free days in patients with acute respiratory distress syndrome due to community-acquired pneumonia.
Methods:
The ventilator STrAtegy for coMmunIty acquired pNeumoniA (STAMINA) study is a randomized, multicenter, open-label trial that compares a driving pressure-limiting strategy to the ARDSnet low-positive end-expiratory pressure table in patients with moderate-to-severe acute respiratory distress syndrome due to community-acquired pneumonia admitted to intensive care units. We expect to recruit 500 patients from 20 Brazilian and 2 Colombian intensive care units. They will be randomized to a driving pressure-limiting strategy group or to a standard strategy using the ARDSNet low-positive end-expiratory pressure table. In the driving pressure-limiting strategy group, positive end-expiratory pressure will be titrated according to the best respiratory system compliance.
Outcomes:
The primary outcome is the number of ventilator-free days within 28 days. The secondary outcomes are in-hospital and intensive care unit mortality and the need for rescue therapies such as extracorporeal life support, recruitment maneuvers and inhaled nitric oxide.
Conclusion:
STAMINA is designed to provide evidence on whether a driving pressure-limiting strategy is superior to the ARDSNet low-positive end-expiratory pressure table strategy for increasing the number of ventilator-free days within 28 days in patients with moderate-to-severe acute respiratory distress syndrome. Here, we describe the rationale, design and status of the trial.
Keywords:Extracorporeal membrane oxygenationPneumoniaPositive pressure respirationRespiration, artificialRespiratory distress syndromeVentilator-induced lung injurySee more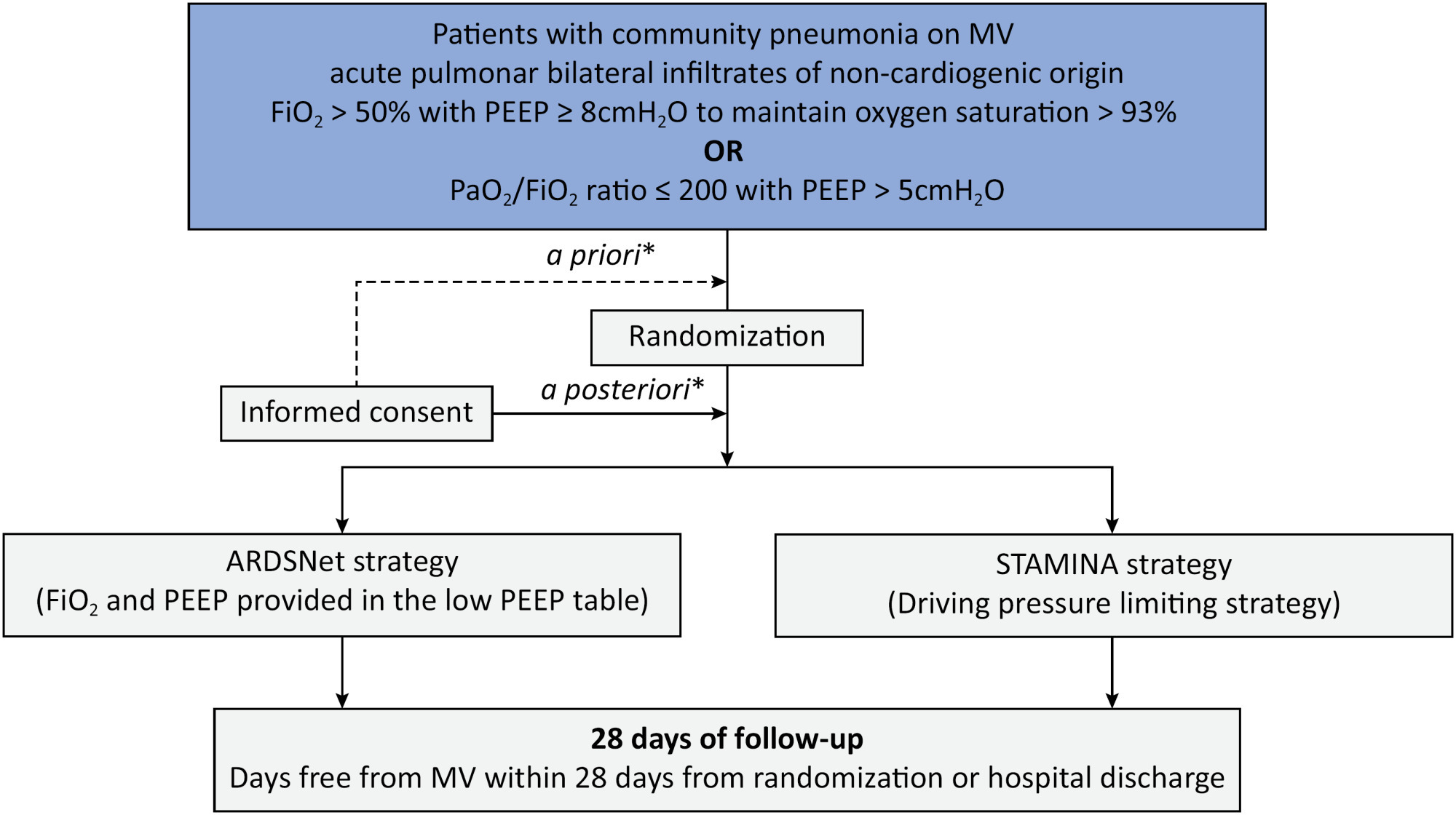
-
Letter to the Editor
To: Posterior reversible encephalopathy syndrome in a child with severe multisystem inflammatory syndrome due to COVID-19
Crit Care Sci. 2023;35(4):429-430
Abstract
Letter to the EditorTo: Posterior reversible encephalopathy syndrome in a child with severe multisystem inflammatory syndrome due to COVID-19
Crit Care Sci. 2023;35(4):429-430
DOI 10.5935/2965-2774.20230322-pt
Views57To the editorWe eagerly read the article by Dominguez-Rojas et al. about a 9-year-old male with a 3-day history of a gastrointestinal infection who underwent explorative abdominal surgery for acute abdomen, which was noninformative.() Postoperatively, the patient developed pneumonia requiring mechanical ventilation.() After extubation, the patient was diagnosed with multisystem inflammatory syndrome in children (MIS-C) […]See more -
Original Article
A comprehensive physical functional assessment of survivors of critical care unit stay due to COVID-19
Crit Care Sci. 2024;36:e20240284en
Abstract
Original ArticleA comprehensive physical functional assessment of survivors of critical care unit stay due to COVID-19
Crit Care Sci. 2024;36:e20240284en
DOI 10.62675/2965-2774.20240284-en
Views57See moreABSTRACT
Objective:
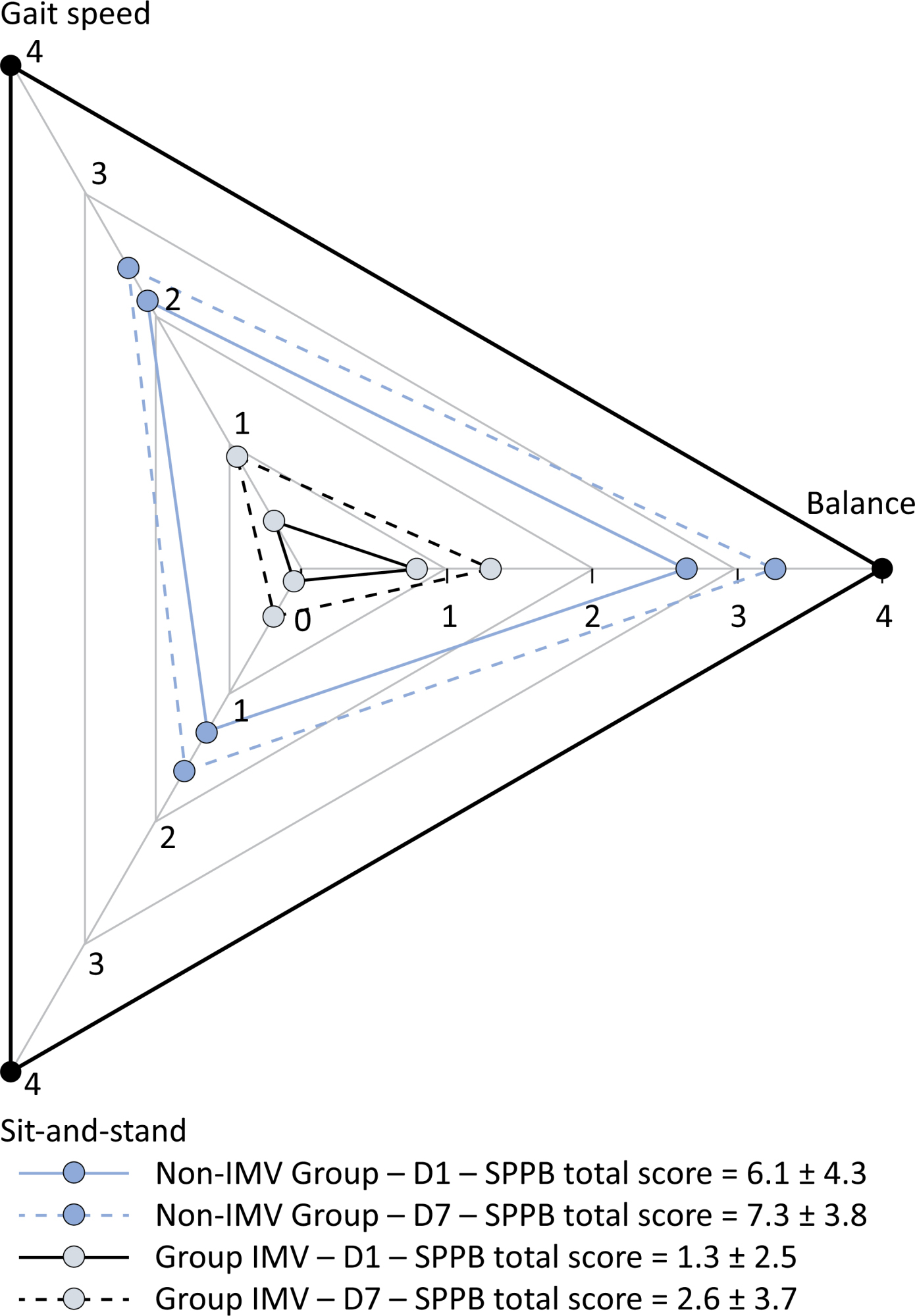
To examine the physical function and respiratory muscle strength of patients – who recovered from critical COVID-19 – after intensive care unit discharge to the ward on Days one (D1) and seven (D7), and to investigate variables associated with functional impairment.
Methods:
This was a prospective cohort study of adult patients with COVID-19 who needed invasive mechanical ventilation, non-invasive ventilation or high-flow nasal cannula and were discharged from the intensive care unit to the ward. Participants were submitted to Medical Research Council sum-score, handgrip strength, maximal inspiratory pressure, maximal expiratory pressure, and short physical performance battery tests. Participants were grouped into two groups according to their need for invasive ventilation: the Invasive Mechanical Ventilation Group (IMV Group) and the Non-Invasive Mechanical Ventilation Group (Non-IMV Group).
Results:
Patients in the IMV Group (n = 31) were younger and had higher Sequential Organ Failure Assessment scores than those in the Non-IMV Group (n = 33). The short physical performance battery scores (range 0 – 12) on D1 and D7 were 6.1 ± 4.3 and 7.3 ± 3.8, respectively for the Non-Invasive Mechanical Ventilation Group, and 1.3 ± 2.5 and 2.6 ± 3.7, respectively for the IMV Group. The prevalence of intensive care unit-acquired weakness on D7 was 13% for the Non-IMV Group and 72% for the IMV Group. The maximal inspiratory pressure, maximal expiratory pressure, and handgrip strength increased on D7 in both groups, but the maximal expiratory pressure and handgrip strength were still weak. Only maximal inspiratory pressure was recovered (i.e., > 80% of the predicted value) in the Non-IMV Group. Female sex, and the need and duration of invasive mechanical were independently and negatively associated with the short physical performance battery score and handgrip strength.
Conclusion:
Patients who recovered from critical COVID-19 and who received invasive mechanical ventilation presented greater disability than those who were not invasively ventilated. However, they both showed marginal functional improvement during early recovery, regardless of the need for invasive mechanical ventilation. This might highlight the severity of disability caused by SARS-CoV-2.
No results found.
Search
Search in:
KEY WORDS
Case reports Child Coronavirus infections COVID-19 Critical care Critical illness Extracorporeal membrane oxygenation Infant, newborn Intensive care Intensive care units Intensive care units, pediatric mechanical ventilation Mortality Physical therapy modalities Prognosis Respiration, artificial Respiratory insufficiency risk factors SARS-CoV-2 Sepsis
Featured Articles
Have your research published in our journal!
Publish your research in a full open access journal with credibility and high scientific and ethical standards.